Herein, we report that cysteine‐derived chiral carbon dots (CDs) can mimic topoisomerase I to mediate topological rearrangement of supercoiled DNA enantioselectively. D ‐CDs can more effectively catalyze the topological transition of plasmid DNA from supercoiled to nicked open‐circular configuration than l ‐CDs. The chiral centre is C-3 of the 2,6-piperidine-2,6-dione ring. The molecule is said to be 'chiral' and the carbon is called a Chiral center. An especially helpful way to recognize chiral carbons is to look for 4 different groups (or atoms) attached to the central carbon: These are but two ways to draw a generic chiral carbon. We defined a chiral center as a tetrahedral carbon with four different substituents. If, instead, a tetrahedral carbon has two identical substituents (two black atoms in the cartoon figure below), then of course it still has a mirror image (everything has a mirror image, unless we are talking about a vampire!).
Isomerism is one of the major areas in organic chemistry which has a broad collection of molecules listed under it. The two types of isomerism are structural isomerism and stereoisomerism. The concept of chirality comes under stereoisomerism. Unlike in structural isomerism, stereoisomerism includes the molecules having the same constitution of atoms but different spatial arrangements. Chirality is the property of a molecule which says its mirror image is non-superimposable with the molecule. The chirality of a certain molecule is determined by the chiral centers present in that molecule. In organic chemistry, chiral centers are called chiral carbons.
Key Areas Covered
1. What is a Chiral Carbon
– Definition, Characteristics
2. How to Identify Chiral Carbons
– Methods to Identify Chiral Carbons in Aliphatic Structure and Ring Structure
3. Why is it Important to Identify Chiral Carbons
Key Terms: Aliphatic Structure, Chirality, Chiral Carbon, Chiral Center, Ring Structure, Stereoisomerism
What is a Chiral Carbon
A chiral carbon is an asymmetric carbon. A carbon atom can have a maximum of four bonds. The chiral carbon is bonded to four different groups. Therefore, it is asymmetric. The carbon atom should always be sp3 hybridized in order to be a chiral carbon. Normally a chiral molecule contains at least one chiral carbon. sp or sp2 hybridized carbon atoms cannot be chiral because they cannot have four different groups around them due to the presence of π-bonds.
Molecules having more than one chiral carbon atoms have two stereoisomers per one chiral carbon. Therefore, that kind of molecules can have more than two stereoisomers. For example, a molecule having two chiral carbons, essentially have four stereoisomers, two per each chiral carbon.
How to Identify Chiral Carbons
How to Identify Chiral Carbon in Aliphatic Structures
Chiral carbon present in a molecule can be identified in two steps as follows.
Step 1
Determine the geometry of the molecule, taking the atom which is assumed to be the chiral carbon in the center.
– If the geometry around the carbon atom is tetrahedral, then it can be a chiral carbon. If the geometry is not tetrahedral, then it is achiral.
Step 2
Determine whether the four groups attached to that carbon atom are different from each other.
– If the carbon atom with the tetrahedral arrangement is attached to four different groups, it is a chiral carbon.
In the above example, the molecule is in tetrahedral geometry and the central carbon atom is attached to four different atoms. Therefore, that carbon is a chiral carbon.
How to Identify Chiral Carbon in Ring Structures
For a ring structure with substitutions, the chirality of a carbon atom can be determined as follows. Consider the following example.
Figure 2: Methyl Cyclohexane
Chiral Carbon Define
Step 1
First, determine whether the groups attached to the carbon atom are different from each other. If they are different, we can guess that it as a chiral carbon. In the above image, the molecule has a hydrogen atom and a methyl group attached to the same carbon atom. But other two groups have formed a ring.
Step 2
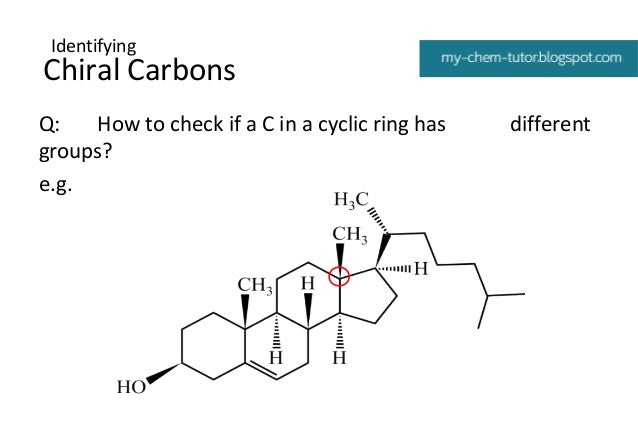
Convert the two groups in the ring into hypothetical groups which are not in a ring. The easiest way to do that is breaking the bond between the atom that is assumed to be the chiral carbon and the adjacent carbon atom on the left side and imagine it as a ligand. Then break the adjacent bond on the right side and imagine it as the other ligand.
Step 3
Now we can determine whether the assumed chiral carbon atom is chiral or achiral. When considering the above molecule, both hypothetical ligands are identical because there are no other substitutions in the ring structure. Therefore, the assumed carbon atom is achiral.
Why is it Important to Identify Chiral Carbons
Identification of chiral carbon explains whether a molecule is chiral or achiral. The determination of the number of chiral carbons present in a molecule is the key to indicate the number of stereoisomers it can have. These stereoisomers are non-superimposable with the molecule. Therefore, it gives information about different molecules which have the same constitution.
Chiral Carbon Center
Summary
This article explains how to identify chiral carbon atoms in a molecule in aliphatic structures or ring structures. The presence of chiral carbon indicates that it has stereoisomers. This is very important in observing the relationships and reactions of molecules.
References:
1.”Chiral center .” OChemPal. N.p., n.d. Web. Available here. 20 June 2017.
2.”Stereochemistry.” The Stereogenic center. N.p., n.d. Web. Available here. 20 June 2017.
Image Courtesy:
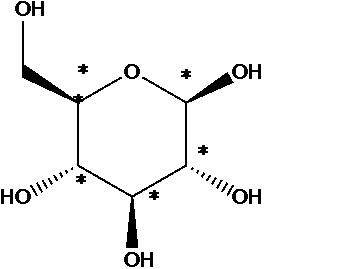
Chiral Carbon Atoms Examples
1.”Chiral” By Isilanes – (Public Domain) via Commons Wikimedia
2. “Methylcyclohexane” By Rhododendronbusch – Own work (Public Domain) via Commons Wikimedia
